August 27, 2024

Burda on Healthcare: The Doctor Is in the House
David W. Johnson, founder and CEO of 4sight Health, likes to say that healthcare will change more in the next 10 years than it has in the past 100 years. I have no reason to doubt him, but as they taught us in journalism school, “Trust, but verify.” If your mother says she loves you, check it out.
While fact-checking this monthly column for 4sight Health, I find myself in a room that was once a bedroom, then an office, and now looks more like a medical exam room.
My home blood pressure cuff and monitor sit on a box of Hammermill printer paper. Two unused boxes of home COVID-19 tests are on my bookshelf. The home pulse oximeter is on my desk near my printer. I bought the pulse oximeter during the pandemic because posting a low blood oxygen level was a sure sign of COVID back in the day. There’s also a home thermometer in my desk drawer for when I’m sure I have COVID but the tests come back negative and my blood oxygen levels are normal.
I have an Apple Watch that rests on a charger. I can strap it on when I need to check my pulse or see whether I have A-fib.
My wife, who is a nurse, helps add to the medical devices around the house. We have two stethoscopes in the bathroom closet across the hall. They’re next to the unopened Cologuard at-home colon cancer screening box. Story for another time.
I have sleep apnea, both kinds, obstructive and central — or, as I like to say, country and western! That’s a whole other device in the bedroom. I sleep with an advanced CPAP machine that transmits my breathing patterns to my sleep doctor.
My self-diagnosis tool kit seems pretty large compared with what my parents had when I was a kid. We had two thermometers. Their use depended on where my mom was going to take our temperature. My sister and I prayed she’d remember which was which.
Yet, I have a feeling my self-diagnosis tool kit will seem minimal to what consumers will be able to do in their own homes in the future to find out if they’re sick, hurt, need to go to the emergency room, drive over to urgent care or make an appointment to see their doctor in-person or on their phone.
Here are a few things I’ve seen in the last few weeks.
Continuously Monitor Your Own Blood Sugar
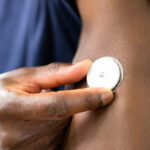 Earlier this month, The Washington Post reported that consumers soon will be able to buy continuous glucose monitors over the counter without a physician’s prescription. The article said the Food and Drug Administration approved two OTC monitors to continuously check a user’s blood sugar level. Two device makers, Dexcom and Abbott, developed the monitors. The FDA approved Dexcom’s continuous glucose monitor in March, per the company’s press release. The FDA approved Abbott’s device in June, per the company’s press release. It’s unclear if either is on the market yet as of this writing.
Earlier this month, The Washington Post reported that consumers soon will be able to buy continuous glucose monitors over the counter without a physician’s prescription. The article said the Food and Drug Administration approved two OTC monitors to continuously check a user’s blood sugar level. Two device makers, Dexcom and Abbott, developed the monitors. The FDA approved Dexcom’s continuous glucose monitor in March, per the company’s press release. The FDA approved Abbott’s device in June, per the company’s press release. It’s unclear if either is on the market yet as of this writing.
Test for Sleep Apnea Without All the Wires
 Also earlier this month, Cardiovascular Business reported that consumers soon will be able to test for sleep apnea at home with a prescription from their doctor. The article said the FDA approved a chest-worn patch to diagnose sleep apnea. Device maker Huxley Medical developed the patch. The device picks up the metrics used to diagnose sleep apnea and transmits them to a clinician for interpretation. The FDA approved Huxley’s home sleep apnea test, dubbed SANSA, on Aug. 7, according to the company’s press release. Again, it’s not clear when patients will start using the tests.
Also earlier this month, Cardiovascular Business reported that consumers soon will be able to test for sleep apnea at home with a prescription from their doctor. The article said the FDA approved a chest-worn patch to diagnose sleep apnea. Device maker Huxley Medical developed the patch. The device picks up the metrics used to diagnose sleep apnea and transmits them to a clinician for interpretation. The FDA approved Huxley’s home sleep apnea test, dubbed SANSA, on Aug. 7, according to the company’s press release. Again, it’s not clear when patients will start using the tests.
A Blood Test to Screen for Possible Colon Cancer
 In late July, NBC News reported that the FDA approved a new blood test to screen for colon cancer as an alternative to the more invasive and more common colonoscopy. A company called Guardant Health developed the new blood test, called Shield. The story said the blood test was 83% effective in finding colon cancer by detecting DNA released by cancerous colon tumors. The FDA approved the test on July 29, according to the company’s press release. Like the sleep apnea device, a doctor would order the test, and a patient would need to get their blood drawn and sent to a lab. But the leap from that to an over-the-counter finger stick version doesn’t seem so far.
In late July, NBC News reported that the FDA approved a new blood test to screen for colon cancer as an alternative to the more invasive and more common colonoscopy. A company called Guardant Health developed the new blood test, called Shield. The story said the blood test was 83% effective in finding colon cancer by detecting DNA released by cancerous colon tumors. The FDA approved the test on July 29, according to the company’s press release. Like the sleep apnea device, a doctor would order the test, and a patient would need to get their blood drawn and sent to a lab. But the leap from that to an over-the-counter finger stick version doesn’t seem so far.
To learn more about this topic, please listen to the Aug. 8, 2024, episode of our 4sight Health Roundup podcast, “Are We Overpaying for an Ounce of Prevention?” on 4sighthealth.com.
Check for Syphilis in the Privacy of Your Own Home
Last week, the Atlanta Journal-Constitution reported that the FDA approved the first-ever home test for syphilis. The FDA approved the test, dubbed First To Know Syphilis Test, on Aug. 16, per the FDA’s press release. A company called NOWDiagnostics developed the test. The test will be available over the counter without a prescription by the end of the year. It’s a finger stick blood test that gives users results in 15 minutes, according to the company’s press release. Syphilis today. Colon cancer tomorrow. Elizabeth Holmes was ahead of her time.
Now, I don’t know much about the life-sciences industry. I know even less about the FDA medical device approval process. I’ve just told you everything I know about home diagnostic tests. Clearly, something is going on. That something appears to be a boom in the home over-the-counter diagnostic test market, or at least a simplified diagnostic test market.
Few, if any, doctors. Few, if any, labs. Minor if any interventions. The power is shifting from incumbent healthcare providers and facilities to consumers in their homes. The doctor literally is in the house.
So, I checked it out, and 4sight Health’s David W. Johnson is right. Healthcare will change more in the next 10 years than in the previous 100 years. This is what he calls the customer revolution in healthcare. For me, I may have to remodel another old bedroom.
Thanks for reading.





